If you run a medical practice, med spa, or any clinic that keeps prescription drugs on-site, there’s a federal deadline approaching that you need to know about: November 27, 2026. That’s when the FDA’s Drug Supply Chain Security Act (DSCSA) compliance becomes mandatory for small dispensers—and yes, that probably includes you.
Don’t panic. This guide will break down everything in plain English, explain what “dispensing” actually means, and show you how to stay compliant without turning your practice upside down.
What’s Actually Happening?
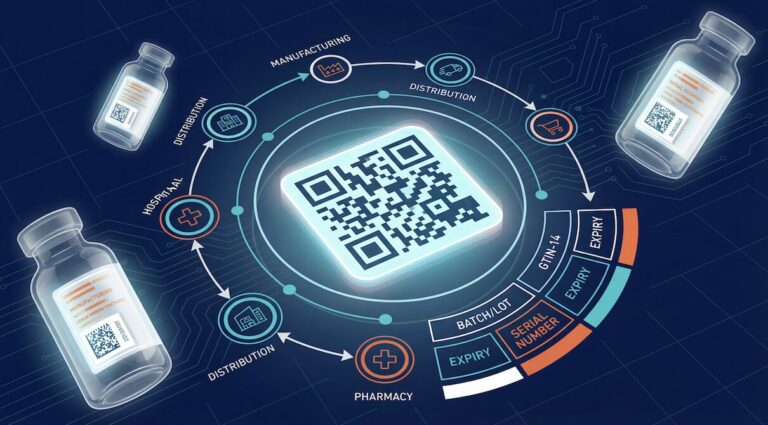
The FDA is enforcing a law called the Drug Supply Chain Security Act (DSCSA). In simple terms, it requires every clinic, pharmacy, and medical practice to track every bottle of prescription medicine electronically—from the manufacturer’s factory all the way to your shelf.
Why? To prevent counterfeit drugs, theft, and contamination from entering the U.S. drug supply. It’s a good goal, but it means new responsibilities for anyone who stocks prescription medications.
The official requirements can be found here:
The Good News: You Have Until November 2026
Originally, the FDA wanted everyone to be 100% digital by 2024. But they realized that small clinics and pharmacies needed more time to purchase software and set up tracking systems.
Here’s what you need to know:
Who Qualifies as a “Small Dispenser”?
Any clinic or pharmacy with 25 or fewer full-time pharmacists or pharmacy technicians. Most private practices, med spas, and specialty clinics fall into this category.
The New Deadline
Because of the small dispenser exemption, you have an extension until November 27, 2026.
What This Means Right Now
You don’t have to implement complex electronic tracking software today, but you must be ready by November 2026. Solutions like TrackTraceRX and similar platforms are designed specifically for this purpose.
The Big Question: Do I Even Need to Worry About This?
This is where things get confusing. Many doctors and practice owners think, “I’m not a pharmacy—does this really apply to me?”
The answer depends on what you do with prescription drugs in your office. Let’s break it down.
Understanding “Prescription” vs. “Dispensing”
What is a Prescription?
A prescription is simply a written or electronic order for medication. When you write a script for a patient to fill at CVS or Walgreens, you’re prescribing—but you’re not dispensing.
Example: Dr. Smith sees a patient with a sinus infection and writes a prescription for amoxicillin. The patient takes the script to their local pharmacy. Dr. Smith is not a dispenser in this scenario.
What is Dispensing?
Dispensing means you physically provide the medication to the patient—either by handing them a bottle to take home or by administering it in your office (like an injection or IV).
The moment you stock prescription drugs in your office, you become a dispenser in the eyes of the FDA.
Example: Dr. Jones runs a med spa and keeps bottles of lidocaine, vitamin B12 injections, and EpiPens on-site. Even though she’s not a pharmacy, she is legally a dispenser because she purchases, stocks, and administers these prescription drugs.
Common Scenarios: Does DSCSA Apply to You?
Scenario 1: “I sell prescription drugs to my patients to take home.”
Answer: YES, you need DSCSA compliance.
If you hand a patient a bottle of medication to take home (even if it’s just a few items), you are classified as a dispenser. You must:
- Verify your suppliers are licensed
- Receive digital tracking data for every product
- Keep those records for 6 years
Scenario 2: “I don’t sell drugs—I just give injections or IVs in the office.”
Answer: IT DEPENDS.
If you administer prescription drugs (like Botox, IV vitamins, or lidocaine injections), you are still a dispenserunder the law. Why? Because you purchased and took ownership of those drugs from a wholesaler.
Even if you never hand a bottle to a patient, you still need to:
- Receive and store the digital tracking data for every drug you purchase
- Maintain records for 6 years
The only way you don’t need DSCSA: If you never stock, buy, or touch a prescription drug. For example, if you only write paper prescriptions for patients to fill elsewhere, you’re off the hook.
Scenario 3: “I keep samples, vaccines, or emergency medications in the office.”
Answer: YES, you need DSCSA compliance.
If your practice keeps any prescription drugs on-site—even if you give them away for free or use them during procedures—you are legally a dispenser.
Common examples:
- Lidocaine for numbing
- EpiPens for emergencies
- Vaccines (flu shots, etc.)
- IV medications or compounded drugs
- Botox, Dysport, or other injectables
- Sample medications from pharmaceutical reps
If you stock it, you’re responsible for tracking it.
“But I’m a Doctor, Not a Pharmacy!”
We hear this all the time. And you’re right—you’re not running a retail pharmacy. But here’s the thing:
The FDA doesn’t care what your sign says. They care what you do with prescription drugs.
If you only write prescriptions, you’re in the clear. But the moment you buy a bottle of lidocaine, an EpiPen, or a box of vaccines to keep in your office, you are legally a dispenser.
The FDA requires you to prove those drugs came from a licensed, authorized trading partner—and a digital tracking solution is the easiest way to document that proof.
What You Need to Do Before November 2026
Step 1: Determine If You’re a Dispenser
Ask yourself:
- Do I stock any prescription drugs in my office?
- Do I administer injections, IVs, or other prescription medications?
- Do I hand patients prescription drugs to take home?
If you answered “yes” to any of these, you’re a dispenser.
Step 2: Choose a DSCSA Compliance Solution
You’ll need software that can:
- Receive and store electronic product tracking data
- Verify your suppliers are licensed
- Maintain records for 6 years
Popular options include TrackTraceRX and similar platforms designed for small dispensers.
Step 3: Work with Your Suppliers
Make sure your wholesalers and distributors are providing you with the required electronic tracking data (called “Transaction Information, Transaction History, and Transaction Statement” or T3 data).
Step 4: Train Your Staff
Your team needs to understand the new process for receiving shipments, verifying data, and maintaining records.
Step 5: Mark Your Calendar
Set a reminder for mid-2026 to ensure everything is in place well before the November 27 deadline.
What Happens If You Don’t Comply?
The FDA takes drug supply chain security seriously. Non-compliance can result in:
- Warning letters
- Fines
- Suspension of your ability to purchase prescription drugs
- Legal action in severe cases
The good news? You still have time. Start planning now, and compliance will be straightforward.
Final Thoughts
The DSCSA might sound intimidating, but it’s really about one thing: proving your prescription drugs are legitimate and safe. If you stock medications in your office—whether you’re a dermatologist, med spa owner, or family practice—you’re a dispenser, and you need to be ready.
The bottom line:
- If you only write prescriptions → You’re fine.
- If you stock, administer, or sell prescription drugs → You need DSCSA compliance by November 27, 2026.
Don’t wait until the last minute. Research your options, talk to your suppliers, and choose a tracking solution that fits your practice. Your patients—and the FDA—will thank you.
Need help getting started? Reach out to DSCSA compliance providers like TrackTraceRX, or consult with your pharmaceutical wholesaler about their electronic data systems. The sooner you start, the smoother the transition will be.
Stay compliant. Stay safe. Modernize your practice the right way.