We Handle ALL Your DSCSA Requirements
We Simplify Your DSCSA Challenges. Get in Touch and Watch our Demo.
- The easiest DSCSA Track & Trace Interoperable system to use.
- Integration to over 100,000+ Trading Partner Locations.
- Trading Partner Onboarding. (Included Free)
- FDA Audit Support.
- 100% EPCIS Graded Data Exchange.
- VRS & Authorized Trading Partner (ATP) Ready.
- Tracing Request Ready.
- ERP or any System Integration.
- Multi-Scanner with Built-in Augmented Reality (AR)
- Standard Operating Procedure (SOP) Template
- Award-Winning Company.
- And Much More…
GET IN TOUCH
Simple to use and elegant Dashboard
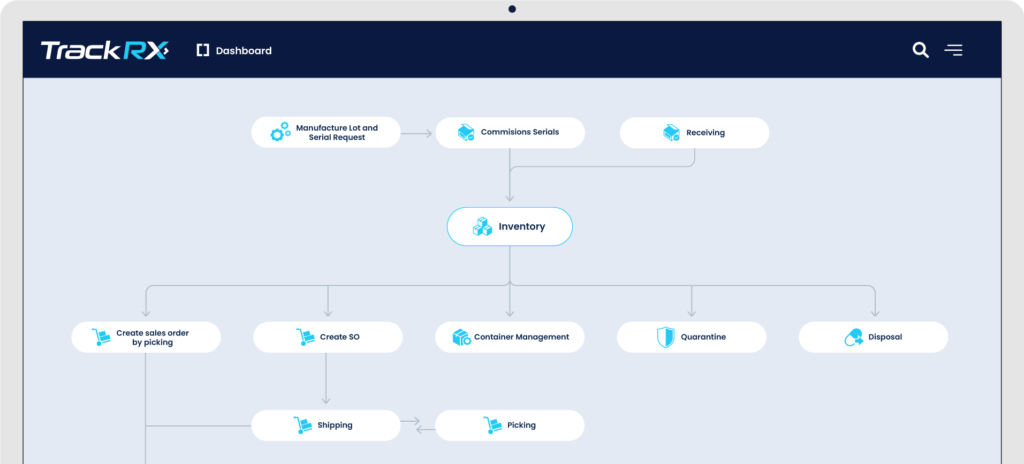
“Easiest system I have ever used!”
“TrackTraceRX provided a single training session, and my entire staff was up to speed. Every other demo we watched from other companies had complex menus we would have had to memorize, but with TrackTraceRX, we just followed the arrows on the dashboard! It was incredibly easy! Moreover, they contacted every single one of my trading partners to integrate with. I was receiving and sending the required compliance data in no time. A complete game changer. ”

Jose Cardenas, CEO of Medpoint Inc.
“We saved 30 minutes per shipment”
“Since implementing the RapidRX smartphone solution for receiving pharmaceutical products in our warehouse, our operations have reported saving an impressive 30 minutes per shipment. The most remarkable aspect is that the RapidRX mobile scanner has completely eliminated error rates. The mobile scanner functions as a comprehensive warehouse terminal, elevating our efficiency and accuracy to an entirely new level.”
- We integrated our Microsoft Great Plain ERP with the TrackTraceRX system for a seamless complete workflow synergy.
- We scanned 66 pallets in under two minutes from distances up to half a meter. This took us to the next level of our operations.

Marcus Koch, GM/COO Johali Medical
About the DSCSA:
Achieve complete traceability with the below Features:
- Cloud Track & Trace Portal.
- Product Verification with VRS.
- Product Tracing.
- Product Recalls
- Authorized Trading Partner Management (ATP)
- API Integration
- Trading Partner Onboarding
- EPCIS Exception Handling
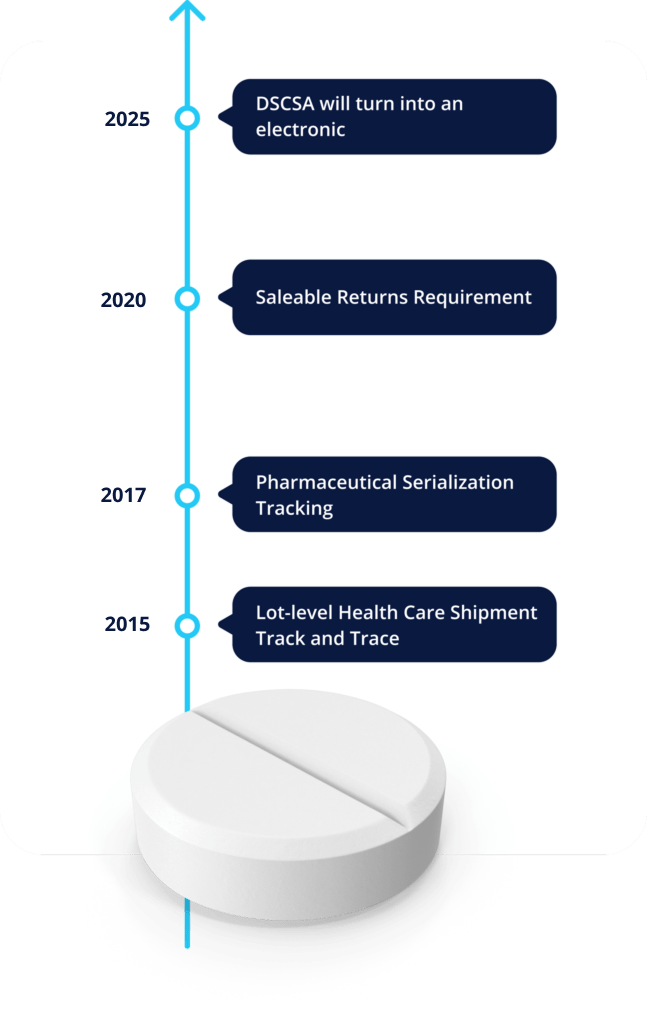
Are you ready for the drug supply chain security act?
Answer the checklist and prepare your business for the future of traceability.
- Understand the legislation.
- Assist your trading partners to ensure they know the legislation.
- Provide product traceability (manufacturers, wholesale distributors, repackagers and dispensers).
- Make sure there is a system in place for identifying and handling illegitimate product.
- Confirm that your trading partners are authorized.
- Report licensure (third-party wholesale distributors and logistics providers).
RapidRX the Future of Supply chain!
We are the first solution provider the provides a scanner with Multi-Scan and Augmented Reality (AR) capable device to help meet the DSCSA requirements.
- Easily verify if a shipment matches track & trace data in real-time using augmented reality.
- Multi-Scan 480 barcodes in one minute. We provide the best serialization scanner!
RapidRX Multiscanner: An extension of the TrackRX Portal
The RapidRX does everything the TrackRX portal giving you the best mobility solution on the market.

We have customers using our solutions for over 15 years!
Join our clients



