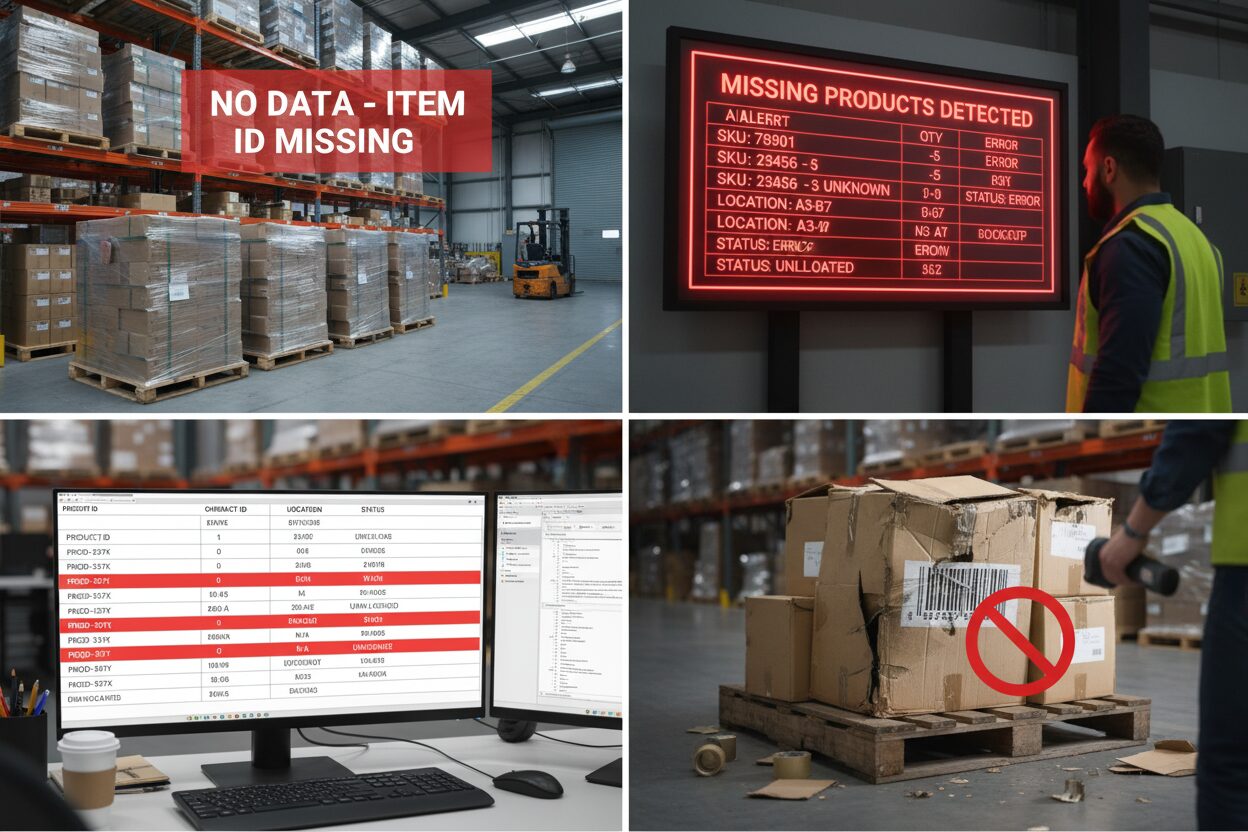
The pharmaceutical supply chain has undergone a fundamental transformation since the Drug Supply Chain Security Act (DSCSA) mandated enhanced traceability and serialization requirements. At the center of this evolution lies the Electronic Product Code Information Services (EPCIS) standard—a sophisticated framework that enables secure, interoperable exchange of serialized product data between trading partners throughout the pharmaceutical distribution network.
Yet even with advanced digital infrastructure and standardized protocols, the reality of pharmaceutical distribution means that exceptions are not just possible—they’re inevitable. A corrupted EPCIS transmission, mismatched serial numbers, damaged barcodes, or simple human error can instantly disrupt the seamless flow of compliant products through the supply chain. When these exceptions occur, they create immediate operational challenges that can cascade throughout the distribution network, affecting everything from inventory management to patient access to critical medications.
This is why EPCIS exception handling has evolved beyond a mere technical necessity into a critical operational discipline that directly impacts patient safety, regulatory compliance, and the trust that binds trading partners together. Organizations that master exception handling don’t just solve compliance problems—they build competitive advantages through operational resilience, enhanced partner relationships, and superior supply chain performance.
The Strategic Imperative: Why Exception Handling Excellence Matters
In today’s pharmaceutical landscape, the cost of exception handling failures extends far beyond immediate operational disruptions:
Regulatory Risk: The FDA’s Enhanced Drug Distribution Security guidance makes clear that trading partners must resolve exceptions promptly and maintain comprehensive documentation. Failure to do so can result in compliance violations, warning letters, and potential enforcement actions.
Operational Impact: Products under exception cannot be distributed until resolution is complete, creating inventory bottlenecks, delayed shipments, and potential stockouts that directly affect patient care.
Financial Consequences: Exception handling consumes significant resources—from dedicated staff time to quarantine space to potential product write-offs. Organizations with poor exception management can see costs spiral quickly.
Partnership Strain: Repeated exceptions or slow resolution times can damage trading partner relationships, leading to lost business opportunities and reduced supply chain flexibility.
Patient Safety: Ultimately, ineffective exception handling can delay patient access to needed medications, making this not just a business issue but a public health imperative.
Understanding EPCIS Exception Categories: A Comprehensive Framework
Effective exception management begins with understanding the distinct types of exceptions that can occur and their unique characteristics, risks, and resolution requirements:

1. Product, No Data: The Physical-Digital Disconnect
Definition: This exception occurs when physical pharmaceutical products arrive at a receiving location, but the corresponding EPCIS transaction information is missing, incomplete, or doesn’t match the physical inventory.
Common Scenarios:
-
Transmission Failures: EPCIS files fail to transmit due to network issues, system downtime, or configuration problems
-
Timing Mismatches: Products arrive before EPCIS data due to different transportation and communication schedules
-
Partial Shipments: Only portion of expected products arrive, but EPCIS file reflects full shipment
-
Aggregation Errors: Case-level products present but not properly associated with pallet-level data
Business Impact:
-
Products must be quarantined immediately upon discovery
-
Cannot be released into sellable inventory until data is provided or alternative resolution achieved
-
Creates inventory accuracy issues and potential financial discrepancies
-
May require manual verification processes that are time-intensive and error-prone
Example: A distributor receives 50 cases of a popular diabetes medication. The physical products are present and appear legitimate, but no EPCIS file has been received from the manufacturer. Without the serialized transaction data, the distributor cannot verify the authenticity of the products or legally distribute them to downstream customers.
2. Data, No Product: The Digital-Physical Gap
Definition: This exception occurs when EPCIS transaction information indicates that specific products should be present, but they are physically missing from the shipment.
Common Scenarios:
-
Picking Errors: Products scanned during order fulfillment but not physically included in shipment
-
Transit Losses: Products lost, stolen, or damaged during transportation
-
Inventory Discrepancies: Systematic errors in warehouse management systems leading to phantom inventory
-
Shipping Mistakes: Products accidentally included in wrong shipments or delivered to incorrect locations
Business Impact:
-
Creates immediate inventory discrepancies requiring investigation and reconciliation
-
May indicate security breaches, theft, or systematic process failures
-
Requires coordination between multiple parties to determine product location and disposition
-
Can trigger suspect product investigations if discrepancies suggest diversion or counterfeiting
Example: An EPCIS file indicates that 1,000 units of a high-value oncology medication were shipped, but physical count reveals only 950 units present. The 50-unit discrepancy must be investigated immediately to determine whether products were diverted, lost in transit, or never actually shipped.
3. Data Issues: The Information Integrity Challenge
Definition: This category encompasses problems with the quality, accuracy, or format of EPCIS data itself, even when the correct amount of product is present.
Common Data Problems:
-
GTIN Errors: Wrong or outdated Global Trade Item Numbers that don’t match physical products
-
Serial Number Issues: Duplicate, missing, or incorrectly formatted serial numbers
-
GLN Problems: Invalid or incorrect Global Location Numbers for trading partner identification
-
Date Discrepancies: Mismatched lot numbers, expiration dates, or manufacturing dates
-
Format Violations: EPCIS files that don’t conform to GS1 standards or implementation guidelines
Business Impact:
-
Can trigger automated verification failures in downstream systems
-
May prevent proper product identification and tracking throughout supply chain
-
Creates audit trail gaps that complicate regulatory compliance
-
Requires manual intervention and data correction processes
Example: A pharmacy receives an EPCIS file with serial numbers that contain invalid characters not conforming to GS1 standards. While the physical products are correct, the data formatting errors prevent the pharmacy’s system from processing the transaction information, requiring manual correction before the products can be dispensed.
4. Damaged Product: The Physical Integrity Challenge
Definition: This exception occurs when products are physically present and EPCIS data is available, but physical damage prevents proper identification, verification, or aggregation.
Common Damage Scenarios:
-
Barcode Damage: 2D data matrix codes that are scratched, torn, or otherwise unreadable
-
Label Issues: Missing, misplaced, or illegible product identification labels
-
Packaging Compromise: Damaged cases, bottles, or containers that may affect product integrity
-
Aggregation Disruption: Damaged shrink wrap or case packaging that prevents inference of contents
Business Impact:
-
May require product quarantine pending quality assessment
-
Can prevent automated scanning and verification processes
-
May necessitate manual verification using alternative methods
-
Could indicate transportation or handling problems requiring investigation
Example: A pallet of blood pressure medications arrives with damaged shrink wrap and several cases showing impact damage. While the EPCIS data is present and accurate, the physical damage prevents automated scanning of case-level barcodes, requiring manual verification of contents before the products can be processed.
Root Cause Analysis: Understanding the Why Behind Exceptions
Effective exception prevention requires understanding the underlying causes that create these disruptions. Industry analysis reveals that exceptions typically trace back to three primary categories of failure:
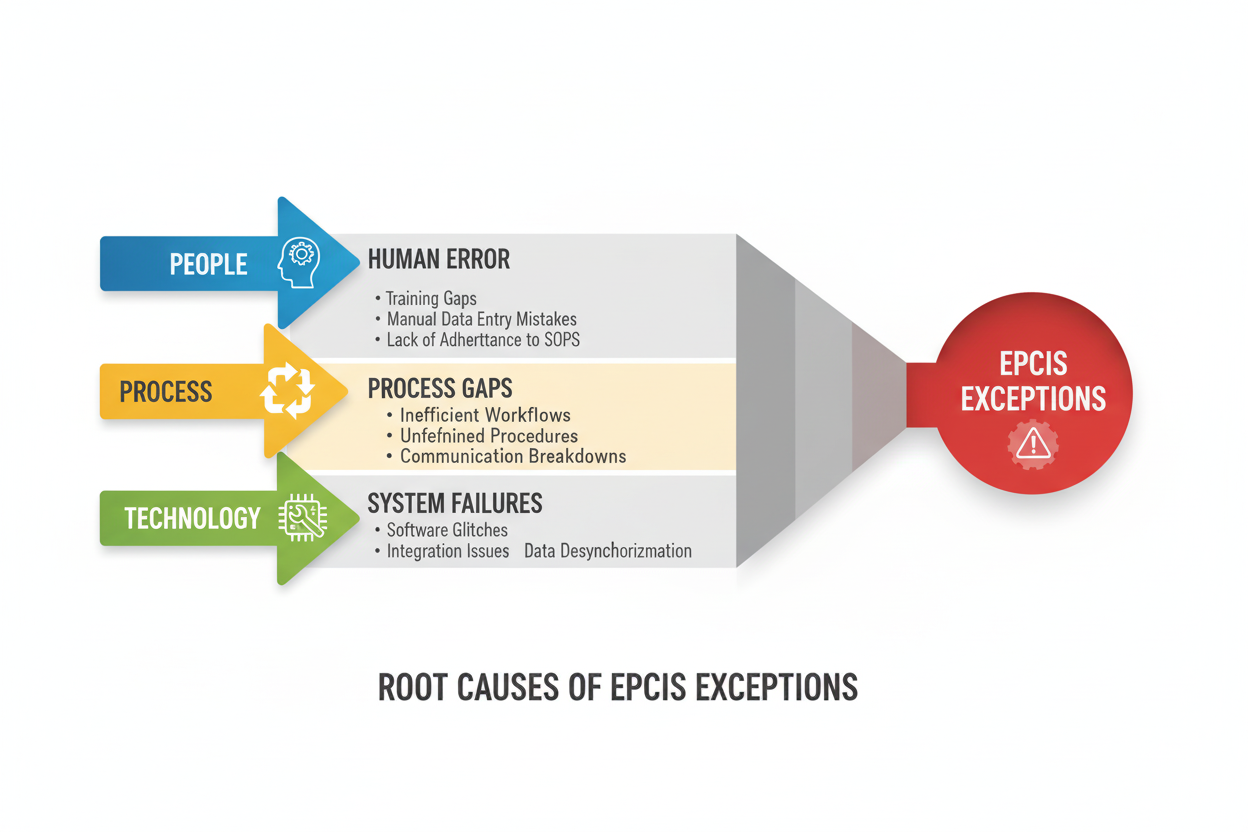
Human Error Factors: The People Dimension
Data Entry Mistakes:
-
Manual transcription errors when entering GTINs, serial numbers, or lot information
-
Typographical errors in system interfaces during order processing
-
Incorrect product selection during picking operations
-
Misreading of product labels or barcodes during scanning
Process Deviations:
-
Failure to follow standard operating procedures during picking and packing
-
Incomplete scanning of all products in a shipment
-
Interruptions in workflow leading to missed steps or incomplete processes
-
Inadequate verification procedures before shipment dispatch
Training Gaps:
-
Insufficient understanding of DSCSA requirements and implications
-
Lack of familiarity with EPCIS data standards and formats
-
Inadequate training on exception identification and initial response procedures
-
Poor understanding of the importance of data accuracy in pharmaceutical distribution
Technology and System Issues: The Infrastructure Dimension
Connectivity Problems:
-
Network failures preventing EPCIS file transmission
-
AS2, SFTP, or API configuration issues blocking data exchange
-
Expired security certificates disrupting authenticated communications
-
Failed message delivery notifications (MDNs) creating uncertainty about transmission success
Integration Challenges:
-
Incompatible data formats between trading partner systems
-
ERP and WMS integration failures causing incomplete data synchronization
-
Version control issues with EPCIS implementation guidelines
-
System timing issues causing data and product arrival mismatches
Hardware Failures:
-
Barcode scanner malfunctions leading to incomplete or inaccurate reads
-
Server downtime preventing EPCIS file generation or transmission
-
Storage system failures corrupting transaction data
-
Network infrastructure problems disrupting real-time communications
Process and Training Gaps: The Organizational Dimension
Communication Breakdowns:
-
Inconsistent exception notification procedures between trading partners
-
Lack of standardized communication templates and protocols
-
Unclear escalation procedures for complex or time-sensitive exceptions
-
Inadequate coordination between different functional teams
Documentation Deficiencies:
-
Incomplete or inaccurate standard operating procedures
-
Lack of clear guidelines for different exception types
-
Insufficient documentation of resolution procedures and best practices
-
Poor record-keeping practices that complicate audit readiness
Organizational Issues:
-
Inadequate staffing for exception handling activities
-
Lack of dedicated exception management resources
-
Insufficient cross-training creating single points of failure
-
Poor performance measurement and continuous improvement processes
The Exception Resolution Workflow: A Structured Approach to Problem-Solving
Effective exception handling requires a systematic, time-bound approach that ensures rapid resolution while maintaining compliance integrity and audit readiness:
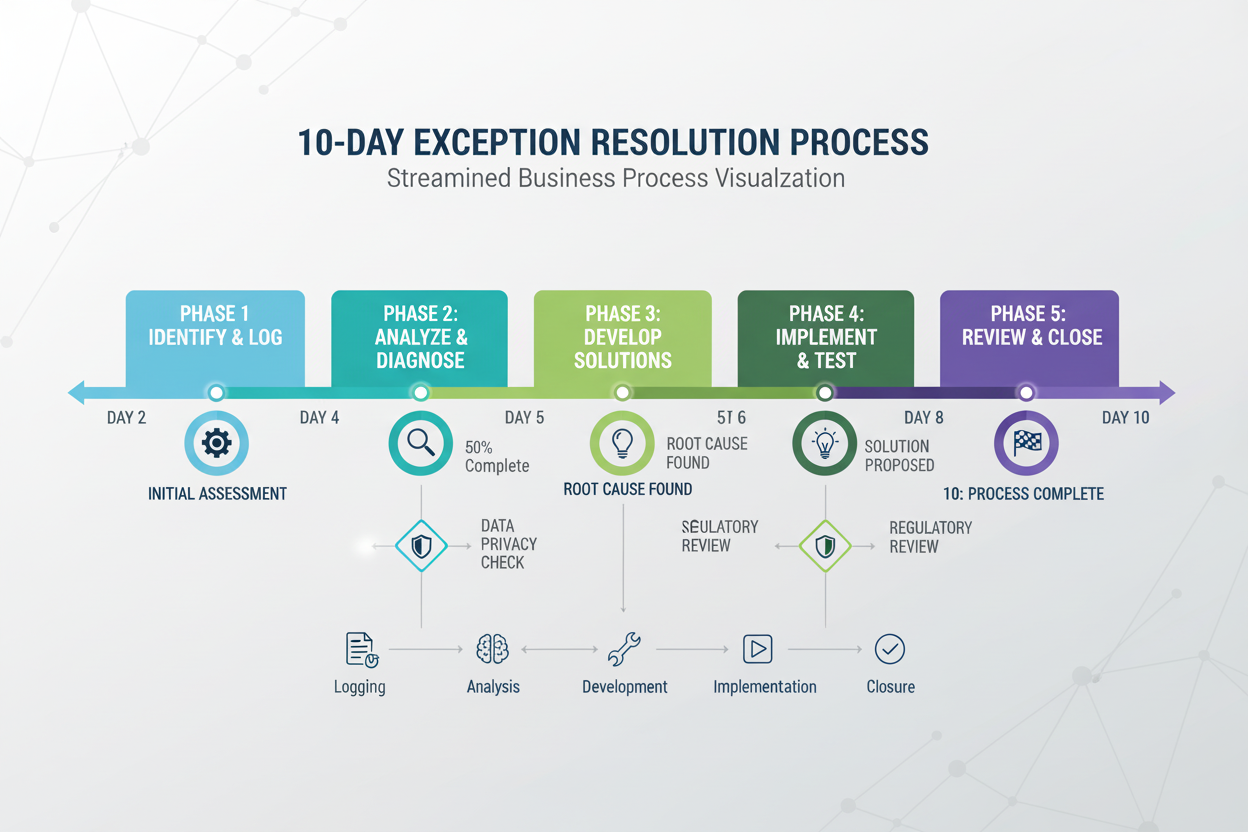
Phase 1: Detection and Initial Assessment (Day 1)
Immediate Response Protocol
The speed and accuracy of initial exception detection often determines the overall success of the resolution process. Leading organizations implement comprehensive detection systems that identify exceptions as early as possible:
Automated Detection Systems:
-
Real-time EPCIS file validation upon receipt
-
Automated reconciliation between expected and received products
-
System alerts for missing or delayed data transmissions
-
Integration with warehouse management systems for immediate notification
Manual Verification Procedures:
-
Systematic physical counts during receiving operations
-
Barcode scanning verification of all products
-
Visual inspection for obvious damage or labeling issues
-
Cross-reference of shipping documents with EPCIS data
Initial Assessment Activities:
-
Categorize exception type using standardized classification system
-
Determine scope and scale of the exception (single package vs. full shipment)
-
Assess urgency based on product type, expiration dates, and customer needs
-
Initiate product quarantine procedures to prevent non-compliant distribution
-
Assign unique tracking identifier for ongoing communication and documentation
Phase 2: Communication and Notification (Day 1-2)
Standardized Communication Protocol
Effective exception resolution is fundamentally collaborative, requiring clear, timely communication between trading partners. Industry best practices emphasize standardized communication formats that ensure all necessary information is provided upfront:
Essential Communication Elements:
-
Standard Subject Line Format: [Sender Company] + [Receiver Company] + [Exception Type] + [Tracking Number]
-
Core Data Requirements: Purchase order number, shipment ID/DESADV, affected NDCs, SSCC numbers
-
Visual Documentation: Barcode scans, photographs of damaged products, screenshots of system errors
-
Scope Definition: Clear description of whether exception affects entire shipment or specific products
-
Contact Information: Designated personnel with authority to resolve the exception
Communication Best Practices:
-
Use dedicated exception handling email addresses monitored by qualified teams
-
Respond to exception notifications within 24 hours with acknowledgment and initial assessment
-
Provide barcode scans rather than manually typed data whenever possible
-
Include photographs of 2D data matrix barcodes with adjacent human-readable information
-
Establish clear escalation procedures for complex or high-priority exceptions
Phase 3: Investigation and Analysis (Day 2-3)
Root Cause Investigation
Thorough investigation is essential for both resolving the immediate exception and preventing future occurrences. This phase requires systematic analysis of all relevant data and processes:
Data Analysis Activities:
-
Review EPCIS transmission logs and message delivery notifications
-
Cross-reference serial numbers against other shipments to identify potential mis-routing
-
Validate product identifiers against manufacturer master data
-
Examine aggregation relationships between packages, cases, and pallets
-
Check for duplicate entries or systematic data errors
Process Investigation:
-
Review picking and packing records for the affected shipment
-
Interview staff involved in order fulfillment and shipping processes
-
Examine transportation and handling records for potential damage or loss
-
Assess system performance during the time period when exception occurred
-
Evaluate compliance with standard operating procedures
Documentation Requirements:
-
Maintain detailed records of all investigation activities
-
Preserve evidence including photographs, barcode scans, and system logs
-
Document timeline of events leading to exception occurrence
-
Record all communication between trading partners during investigation
Phase 4: Resolution Implementation (Day 3-10)
Systematic Problem Resolution
The resolution approach must be tailored to the specific exception type while maintaining compliance with FDA guidance and industry best practices:
For Product, No Data Exceptions:
-
Supplemental EPCIS File: Upstream partner provides missing transaction information using incremental file approach
-
Data Verification: Confirm accuracy of supplemental data against physical products
-
System Updates: Update receiving partner’s systems with corrected transaction information
-
Alternative Documentation: Use of photographs and manual verification in limited circumstances
For Data, No Product Exceptions:
-
Corrective EPCIS File: Remove erroneous serial numbers from transaction records
-
Product Location Investigation: Determine actual location and disposition of missing products
-
Replacement Shipment: Arrange for missing products to be shipped if available
-
Financial Reconciliation: Adjust invoicing and payments to reflect actual products received
For Data Issues:
-
Data Correction Process: Update incorrect information in EPCIS systems using standardized correction procedures
-
File Replacement: Issue corrected transaction files with proper version control
-
System Validation: Verify that corrected data processes successfully through all systems
-
Quality Assurance: Implement additional checks to prevent similar data errors
For Damaged Product:
-
Quality Assessment: Evaluate whether product integrity has been compromised
-
Alternative Verification: Use manual methods when automated scanning is not possible
-
Product Disposition: Determine whether products can be released, returned, or must be destroyed
-
Process Improvement: Address underlying causes of damage during transportation or handling
Phase 5: Documentation and Closure (Day 10+)
Compliance and Continuous Improvement
Proper closure of exceptions ensures regulatory compliance and provides valuable data for preventing future occurrences:
Documentation Requirements:
-
Complete exception resolution record including all investigation steps and findings
-
Preserve all communication between trading partners throughout the process
-
Update internal systems to reflect final resolution and any data corrections
-
Maintain audit-ready files that demonstrate compliance with FDA guidance
Performance Analysis:
-
Measure resolution time against established targets and industry benchmarks
-
Analyze root causes to identify patterns and systemic issues
-
Assess effectiveness of resolution approach and identify improvement opportunities
-
Calculate financial impact including staff time, quarantine costs, and potential lost sales
Continuous Improvement Activities:
-
Update standard operating procedures based on lessons learned
-
Provide additional training to staff involved in exception handling
-
Implement preventive measures based on root cause analysis
-
Share insights with trading partners to improve overall supply chain performance
Best Practices for Exception Prevention: Proactive Strategies for Supply Chain Excellence
While robust resolution capabilities are essential, prevention remains the most effective strategy:
Upstream Prevention Strategies: Seller-Side Excellence
Pre-Shipment Reconciliation:
Organizations shipping pharmaceutical products can dramatically reduce exceptions through systematic verification before products leave their facilities:
-
Data-Product Reconciliation: Verify that EPCIS transaction information accurately reflects physical products being shipped
-
Quality Control Checkpoints: Implement multiple verification steps throughout picking and packing processes
-
Automated Validation: Use system controls that prevent shipment when data-product mismatches are detected
-
Final Verification: Conduct comprehensive review of shipment contents against EPCIS files before dispatch
System Optimization:
-
EPCIS Transmission Validation: Confirm successful file transmission with message delivery notifications (MDNs) before shipment
-
Master Data Accuracy: Maintain current and accurate product information including GTINs, GLNs, and aggregation relationships
-
Integration Testing: Regularly verify data exchange accuracy with all trading partners
-
Performance Monitoring: Track exception rates and resolution times to identify improvement opportunities
Staff Training and Development:
-
DSCSA Education: Comprehensive training on regulatory requirements and compliance implications
-
Process Training: Detailed instruction on proper scanning, aggregation, and data handling procedures
-
Error Recognition: Training staff to identify and report potential exceptions before they impact customers
-
Continuous Learning: Regular updates on new requirements, best practices, and lessons learned
Downstream Prevention Strategies: Buyer-Side Preparedness
Receiving Excellence:
Organizations receiving pharmaceutical products can implement their own prevention measures to catch and address potential exceptions early:
-
Immediate Verification: Systematic checking procedures for all incoming shipments
-
EPCIS Validation: Real-time verification of transaction information upon receipt
-
Exception Detection: Automated systems that identify discrepancies as they occur
-
Staff Competency: Training receiving personnel on proper barcode scanning techniques
Data Management:
-
Maintain robust backup and recovery systems for EPCIS data
-
Implement real-time monitoring of data transmission success
-
Use automated reconciliation tools to identify discrepancies quickly
-
Establish regular audits of data accuracy and completeness
Regulatory Considerations and FDA Guidance:
The FDA’s Enhanced Drug Distribution Security (EDDS) guidance provides important direction for exception handling:
Key FDA Expectations
-
Three-Day Resolution Timeline: Exceptions should be resolved within three business days when possible
-
Product Quarantine: Products under exception should not be sold until resolution
-
Documentation Requirements: Maintain detailed records of all exception handling activities
-
Trading Partner Cooperation: Work collaboratively to resolve discrepancies
Compliance Implications
-
Audit Preparedness: Maintain comprehensive documentation for FDA inspections such as a SOP
-
Risk Assessment: Evaluate whether exceptions indicate potential suspect products
-
Verification Requirements: Use appropriate verification methods for different exception types
-
Reporting Obligations: Understand when exceptions may require FDA notification
Measuring Exception Handling Performance: Key Metrics for Continuous Improvement
Effective exception management requires ongoing monitoring and measurement:
Key Performance Indicators
-
Exception Rate: Percentage of shipments experiencing exceptions
-
Resolution Time: Average time to resolve different exception types
-
Root Cause Distribution: Frequency of different exception causes
-
Prevention Effectiveness: Reduction in exception rates over time
Continuous Improvement Metrics
-
Training Effectiveness: Reduction in human error-related exceptions
-
System Performance: Decrease in technology-related exceptions
-
Communication Efficiency: Faster response times and clearer information exchange
-
Cost Impact: Financial impact of exceptions and resolution activities
Building a Culture of Exception Excellence: Organizational Capabilities for Success
Successful EPCIS exception handling requires more than just processes and technology—it requires a culture that prioritizes accuracy, communication, and continuous improvement:
Organizational Elements
-
Leadership Commitment: Executive support for exception handling investments
-
Cross-Functional Teams: Collaboration between operations, IT, and quality assurance
-
Trading Partner Relationships: Strong partnerships built on trust and communication
-
Continuous Learning: Regular review and improvement of exception handling practices
Training and Development
-
Comprehensive Education: Training on DSCSA requirements and exception handling procedures
-
Regular Updates: Ongoing education on new regulations and best practices
-
Hands-On Practice: Simulation exercises to prepare staff for exception scenarios
-
Performance Feedback: Regular assessment and coaching for improvement.
Technology Solutions for Exception Management: Leveraging Innovation for Operational Excellence
Modern technology solutions can significantly improve both exception prevention and resolution:
Advanced Scanning Technologies
-
Vision Scanning Systems: Can capture multiple barcodes simultaneously with higher accuracy
-
Augmented Reality Interfaces: Provide real-time guidance and error detection
-
Mobile Applications: Enable rapid exception reporting and resolution tracking
-
AI-Powered Verification: Automatically detect and flag potential exceptions
Integration Platforms
-
Cloud-Based EPCIS Systems: Facilitate real-time data sharing and reconciliation
-
API-First Architectures: Enable seamless integration with existing warehouse systems
-
Automated Workflow Engines: Streamline exception handling processes
-
Real-Time Monitoring Dashboards: Provide visibility into exception trends and resolution status
Ready to Transform Your Exception Handling Capabilities?
TrackTraceRx offers comprehensive DSCSA compliance solutions that can revolutionize your approach to exception management. From the RapidRx mobile application that provides real-time exception detection and resolution to the full TrackTraceRx platform that automates and optimizes your entire EPCIS workflow, we have the tools and expertise to help you achieve exception handling excellence.
Contact TrackTraceRx Today:
Website: www.tracktracerx.com
Email: info@tracktracerx.com
Phone: 1-(321) 418-7147
Discover how TrackTraceRx can help you:
- Reduce exception rates by up to 75%
- Accelerate resolution times from days to hours
- Automate routine exception handling tasks
- Strengthen trading partner relationships
- Ensure comprehensive FDA compliance